|
#2
July 23rd, 2016, 12:09 PM
| |||
| |||
| Re: Download IIT JEE Question Paper
As you requires I am here giving you sample question paper for IIT JEE advanced exam for preparation of exam. IIT JEE Advanced Exam Paper : 21. The correct combination of names for isomeric alcohols with molecular formula C4H10O is/are (A) tert-butanol and 2-methylpropan-2-ol (B) tert-butanol and 1, 1-dimethylethan-1-ol (C) n-butanol and butan-1-ol (D) isobutyl alcohol and 2-methylpropan-1-ol 25. Hydrogen bonding plays a central role in the following phenomena: (A) Ice floats in water. (B) Higher Lewis basicity of primary amines than tertiary amines in aqueous solutions. (C) Formic acid is more acidic than acetic acid. (D) Dimerisation of acetic acid in benzene. 26. In a galvanic cell, the salt bridge (A) does not participate chemically in the cell reaction. (B) stops the diffusion of ions from one electrode to another. (C) is necessary for the occurrence of the cell reaction. (D) ensures mixing of the two electrolytic solutions. 27. Upon heating with Cu2S, the reagent(s) that give copper metal is/are (A) CuFeS2 (B) CuO (C) Cu2O (D) CuSO4 28. The correct statement(s) for orthoboric acid is/are (A) It behaves as a weak acid in water due to self ionization. (B) Acidity of its aqueous solution increases upon addition of ethylene glycol. (C) It has a three dimensional structure due to hydrogen bonding. (D) It is a weak electrolyte in water. 29. For the reaction: I – + ClO3– + H2SO4 → Cl – + HSO4– + I2 The correct statement(s) in the balanced equation is/are: (A) Stoichiometric coefficient of HSO4– is 6. (B) Iodide is oxidized. (C) Sulphur is reduced. (D) H2O is one of the products. 30. The pair(s) of reagents that yield paramagnetic species is/are (A) Na and excess of NH3 (B) K and excess of O2 (C) Cu and dilute HNO3 (D) O2 and 2-ethylanthraquinol 31. Consider all possible isomeric ketones, including stereoisomers, of MW = 100. All these isomers are independently reacted with NaBH4 (NOTE: stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are 32. A list of species having the formula XZ4 is given below. XeF4, SF4, SiF4, BF4–, BrF4–, [Cu(NH3)4]2+, [FeCl4]2–, [CoCl4]2– and [PtCl4]2–. Defining shape on the basis of the location of X and Z atoms, the total number of species having a square planar shape is 35. Consider the following list of reagents: Acidified K2Cr2O7, alkaline KMnO4, CuSO4, H2O2, Cl2, O3, FeCl3, HNO3 and Na2S2O3. The total number of reagents that can oxidise aqueous iodide to iodine is 38. If the value of Avogadro number is 6.023 × 1023 mol–1 and the value of Boltzmann constant is 1.380 × 10–23J K–1, then the number of significant digits in the calculated value of the universal gas constant is 39. A compound H2X with molar weight of 80 g is dissolved in a solvent having density of 0.4 g ml –1. Assuming no change in volume upon dissolution, the molality of a 3.2 molar solution is 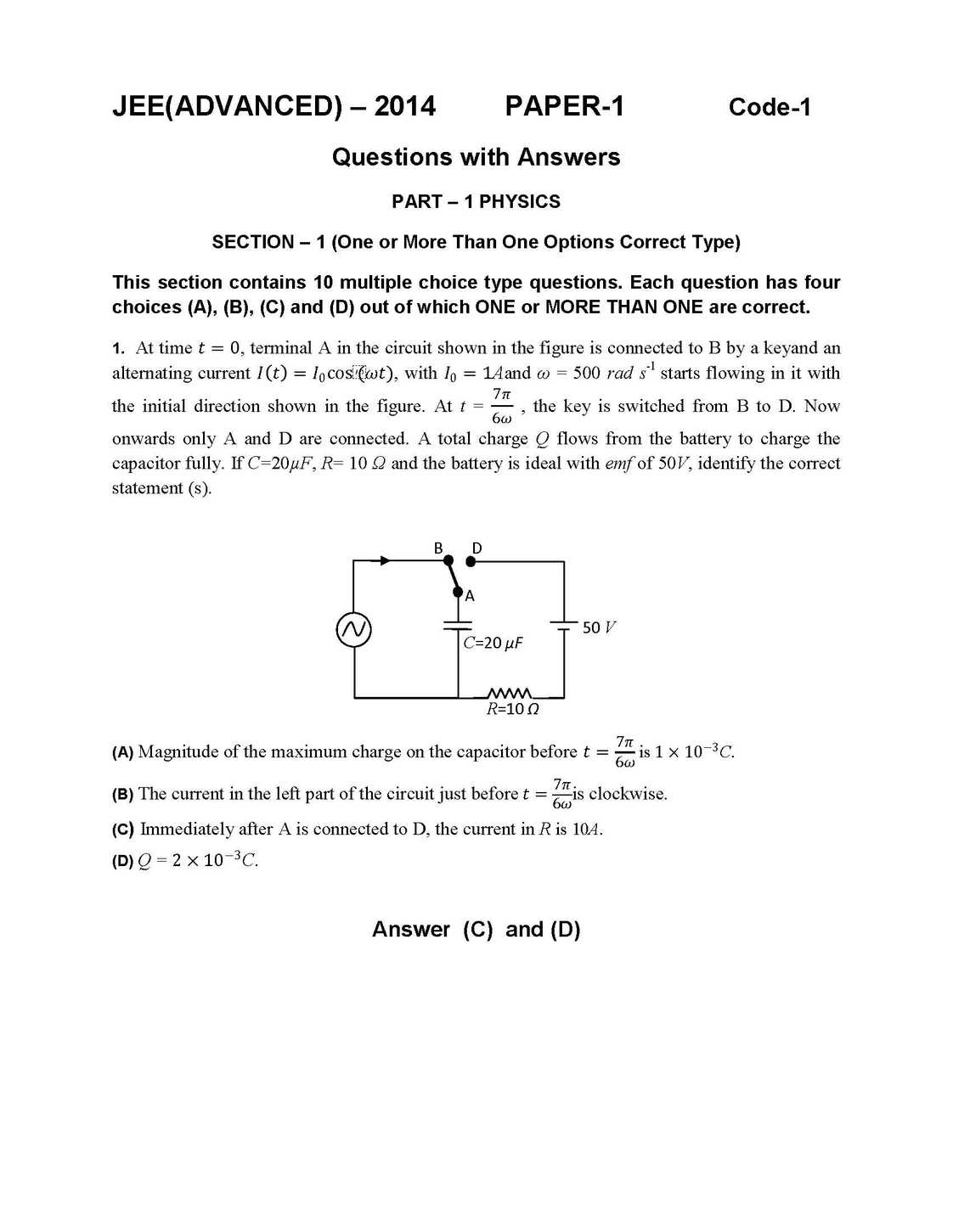  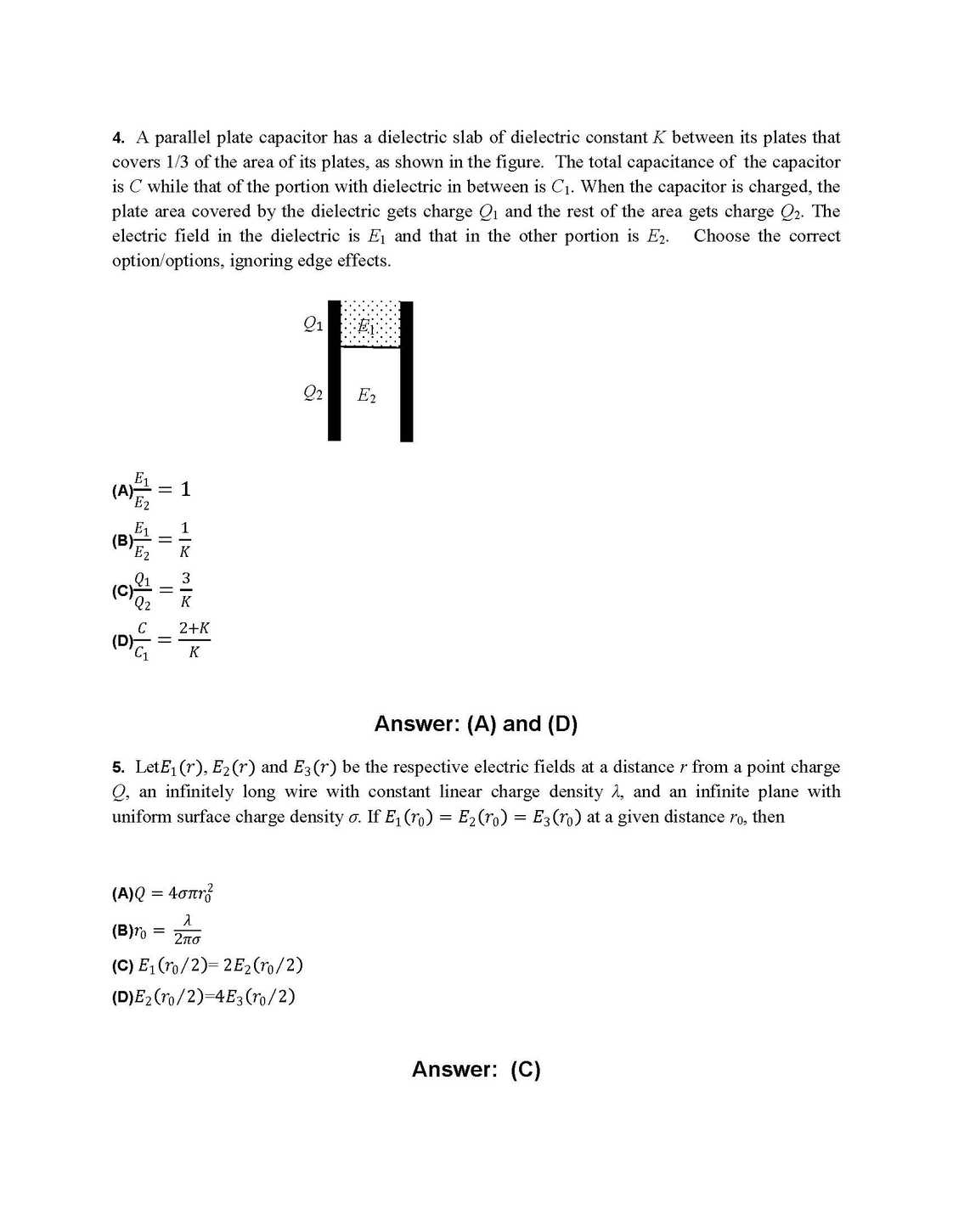 Here is the attachment . |