|
#1
May 13th, 2016, 10:35 AM
| |||
| |||
| HCL Pka in Water
Hi I would like to have the details of the pKA table with respect to the values of the different acids and specifically Hydrochloric Acid? Understanding the best possible utilization of a pKa table will give you the capacity to perceive which corrosive base responses will happen and which won't. This will come up a great deal as you advance through Org 1 and Org 2. 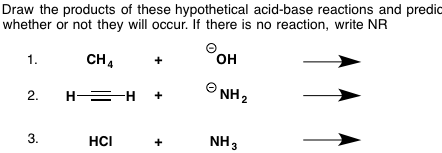 Where to begin with this issue? Keep in mind that a pKa table positions particles all together of their acridity, from emphatically acidic (e.g. HCl with pKa of –8) to feebly acidic (e.g. methane, pKa of ~50). What figures out if or not a corrosive base response will happen in any case? We apply the accompanying standard to corrosive base responses: A more grounded corrosive will have a tendency to respond with a more grounded base to create a weaker corrosive and a weaker base. 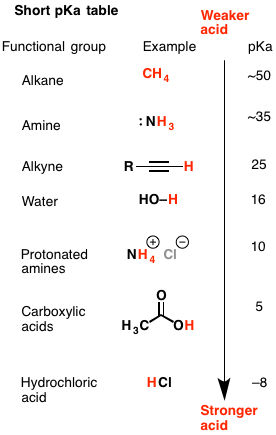 It's sufficiently simple to utilize a pKa table to decide corrosive quality – It can seen initially that H2O (pKa of 15) is a more grounded corrosive than NH3 (pKa of 38). The inquiry is, how would it be decided by base quality? 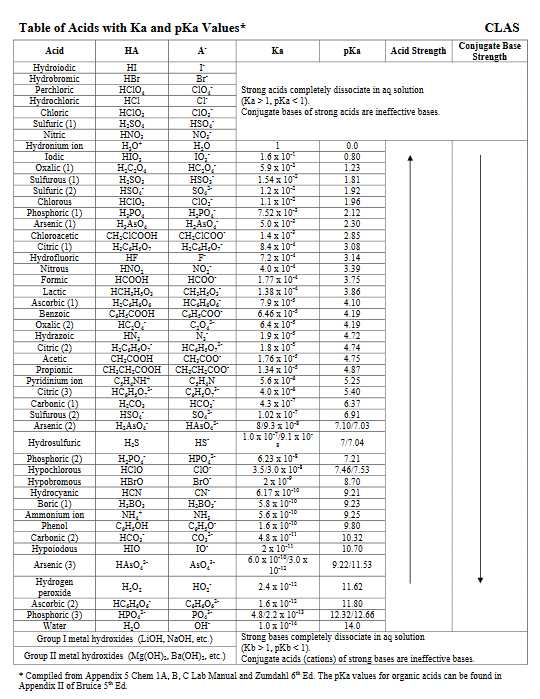 Last edited by Neelurk; April 7th, 2020 at 11:10 AM. |