|
#2
June 8th, 2016, 12:53 PM
| |||
| |||
| Re: Indian Institute of Space Science and Technology Sample Papers
As per your requirement I am here giving you sample test paper for Indian Institute of Space Science and Technology (IIST) entrance exam. IIST sample Paper : 5. In a Young’s double slit experiment, the separation between the slits is 1.0 mm and the distance between the slits and the screen is 1.0 m. The light falling on the slits contains mainly two wavelengths 600 nm and 500 nm. The least distance from the centre of the fringe pattern where the intensity corresponding to one of these wavelengths is zero, is (a) 0.30 mm (b) 0.75 mm (c ) 0.25 mm (d) 1.20 mm 10. The mass density in a nucleus near its center, in units of kg/m3, is in the range (a) 1015 to 1020 (b) 105 to 1010 (c ) 1010 to 1015 (d) 1020 to 1025 11. For the nucleus 216Te, the value of r for which the nucleon density falls to half its value at the centre is in the range (a) 7 to 8 fm (b) 5 to 6 fm (c) 6 to 7 fm (d) 8 to 9 fm 21. The correct order of ligand field strength is (a) H2O < Cl- < CO < NH3 (b) CO < NH3 < Cl- < H2O (c) H2O < CO < NH3 < Cl- (d) Cl- < H2O < NH3 < CO 22. The complex exhibiting a spin-only magnetic moment (s) of 2.87 B.M. is (a) [Co(H2O)3F3] (b) K4[Fe(OH)6] (c) Na2[Cr(NCS)4(NH3)2] (d) K2[MnCl4(H2O)2] 25. Cyclohexanol is converted to Nylon-6 by (a) Na2O/NH2OH/H+/250 OC (b) Cu(250 OC)/NH2OH/H+/250 OC (c) Ag2O/NH2OH/H+/250 OC (d) Cu(250 OC)/NH2OH/250 OC 27. A vessel, fitted with a weightless, frictionless piston of 0.025 m2 area, contains excess con. HCl. The piston moved 1 m outward when 0.075 kg of iron filings were added at 300 K. The solution left behind was found to contain Fe(II). The approximate purity of the iron sample is (a) 50 % (b) 75 % (c) 90 % (d) 40 % 28. A solution at 298 K is separated from the pure solvent by a semi-permeable membrane. Difference in the height of the solution and the solvent is 0.9 m. If Kf and freezing point of the solvent are 30 K kg mol-1 and 250.3 K, respectively, the temperature at which the solution freezes is (a) 250.10 K (b) 250.25 K (c ) 250.20 K (d) 250.05 K IIST sample Test Paper 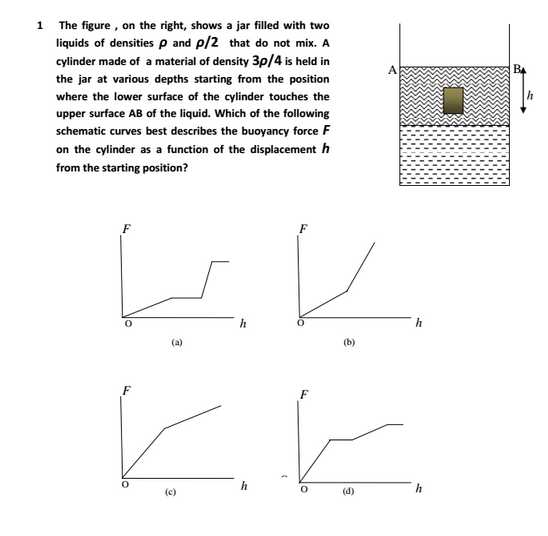 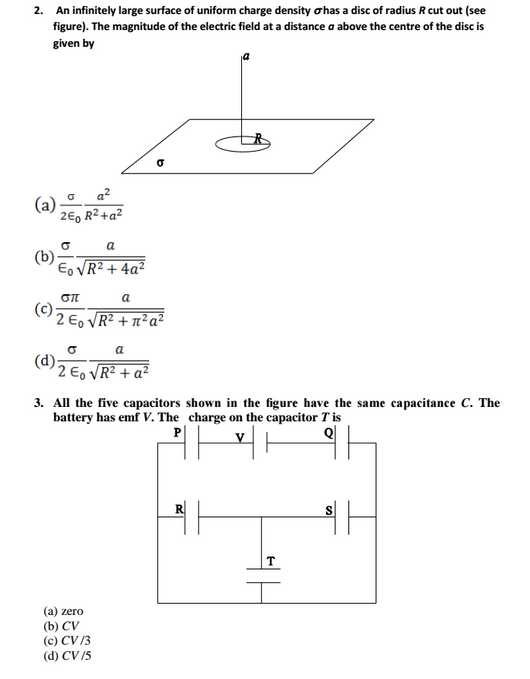  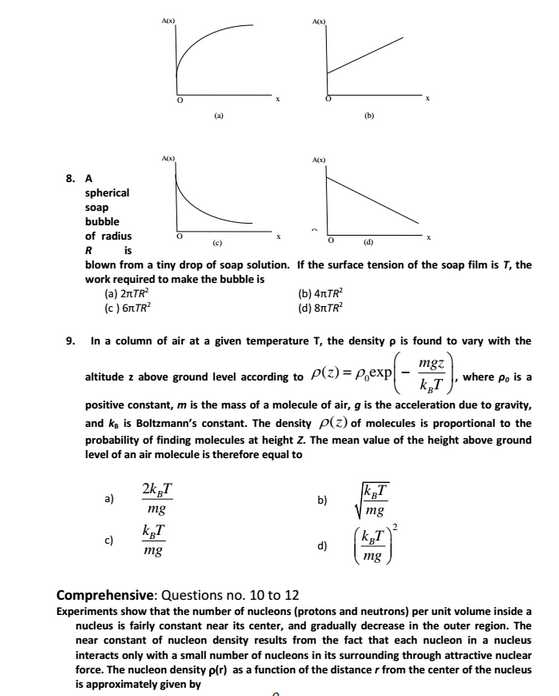 Here is the attachment. |