|
#2
September 10th, 2016, 03:13 PM
| |||
| |||
| Re: Mysore University M Tech
The syllabus of M. Tech Program in Materials Science as offered by University of Mysore is as follows: MSH-1 : Introduction to Materials: 4 Credits (Hard core) Unit-I Materials through ages Materials, Clarification, Crystalline, Amorphous, Glasses; Metals, Alloys, Semiconductors, Polymers Ceramics, Bio-materials Polymers, Blends, Composites. Bulk and Nanomaterials. Quantization effect. Unit-II Crystal Structure Periodic Array of Atoms Crystal Lattice-Lattice Translation Vectors - United-Basis-Symmetry Consideration- Bravis Lattice - Crystal Planes and Millers Indices-Simple Crystal Structure (HCP, FCC, BCC, SC, Diamond). Unit-III Crystal Diffraction and Reciprocal Lattice (Qualitatives) Bragg's Law, Laue Equations, Reciprocal Lattice, Braggs Condition, Brillouin Zones, Atomic Scattering, Geometrical Structure Factor, Experimental X-Ray Diffraction, Methods of Crystal Structure, Laue Method, Rotary Crystal Method, Powder Method or Debye Scherrer Method, Weber Feckner Method. Unit-IV Crystal Chemical Bonding and Imperfection Ionic Crystals, Covalent Crystals, Metallic Crystals Molecular Crystals and Vander Waals Attraction, Hydrogen Bonded Crystals, Mixed or Multiple Bond Crystals. Classification of Imperfections, Concentration of Vacancies (Schottky Defects), Frenkel Defects, Extrinsic vacancies, Color Centers, Dislocations, Dislocation Energies, Dislocation and Shear Strength of Single Crystal, Defects, Grain Boundaries, Staining Faults. MSH-2 :Thermodynamics and Statistical Mechanics 4 Credits (Hard core) Unit I: Kinetic Theory and Gas Laws Kinetic Theory of Matter, Different States of Matter, Concept of Ideal or Perfect Gas, Kinetic Theory of Gases, Expression for the Pressure of a Gas, Kinetic interpretation of Temperature. Unit II: Equation of State Derivation of Gas Equation, Derivation of Gas Laws, Avogadro's Hypothesis, Graham's Law of Diffusion of Gases, Degree of Freedom & Maxwell's Law of Equipartition of Energy, Mean Free Path, Van-der Waals Equation of State, Critical Constants, Corresponding States, Critical Coefficient. Unit III: Laws of Thermodynamics Thermal Equilibrium Concept of Temperature (Zeroth Law of Thermodynamics), Concept of Heat-Heat: A Path Function, Work: A Path Function, Comparison of Heat and Work - First Law of Thermodynamics, Isothermal Process, Adiabatic Process, Isobaric Process, Isochoric Process, Second Law of Thermodynamics, Entropy, Third Law of Thermodynamics. Unit IV: Statistical Thermodynamics Statistical Mechanics, Statistical Equilibrium, Statistical definition of entropy, Gibbs Paradox-Probability Theorems, Statistical Thermodynamics, Maxwell-Boltzmann Distribution Law Maxwell-Boltzmann Distribution & Ideal Gas, Quantum Statistics, Phase Space, Fermi-Dirac Distribution Law, Electron Gas, Bose-Einstein Distribution Law, Photon Gas, Comparison of Three Statistics Bragg Weiler's approximation.Therories of simple liquids-Monte-Carlo Molecular dynamics simulations-Reaction dynamics from microscopic viewpoint. 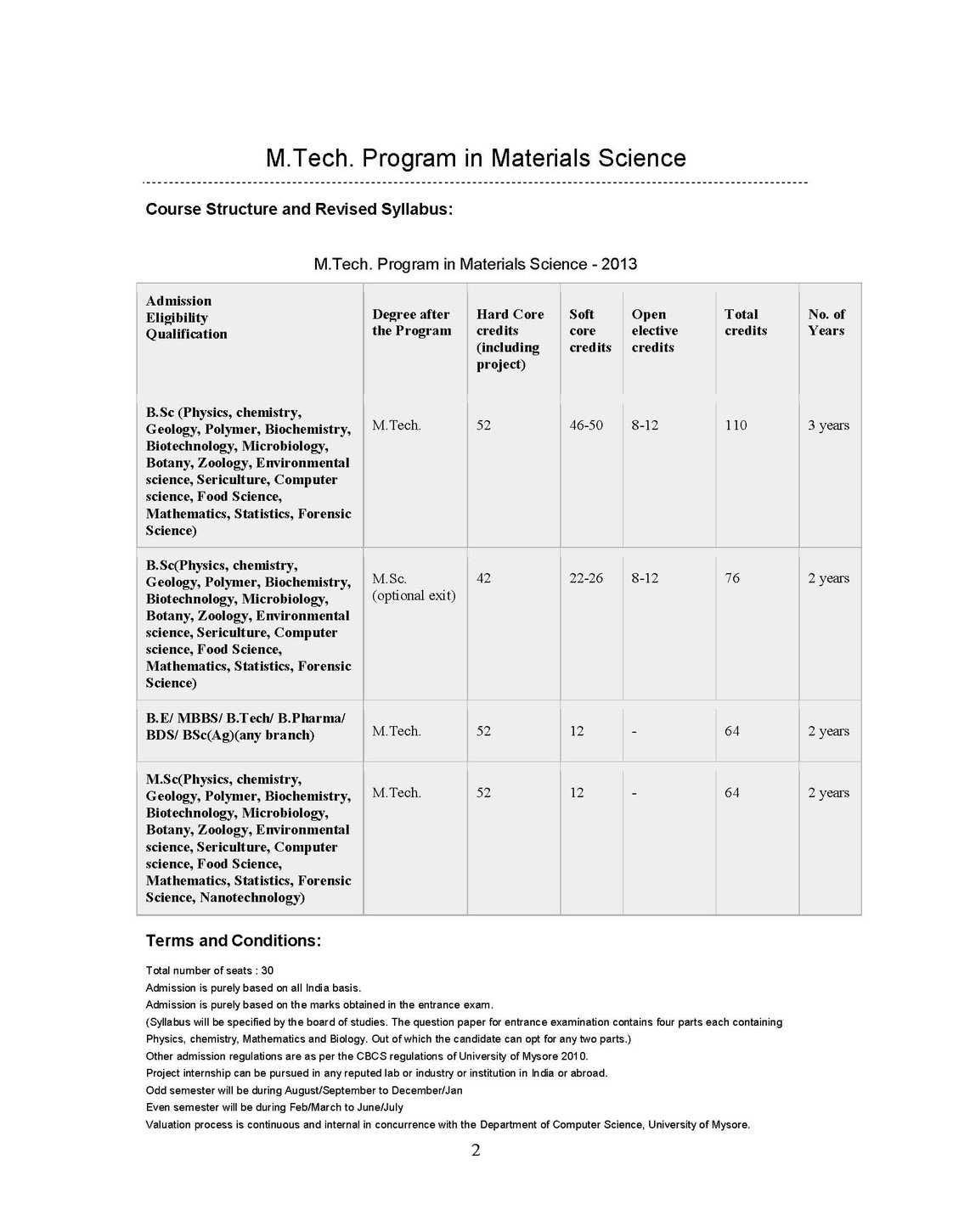 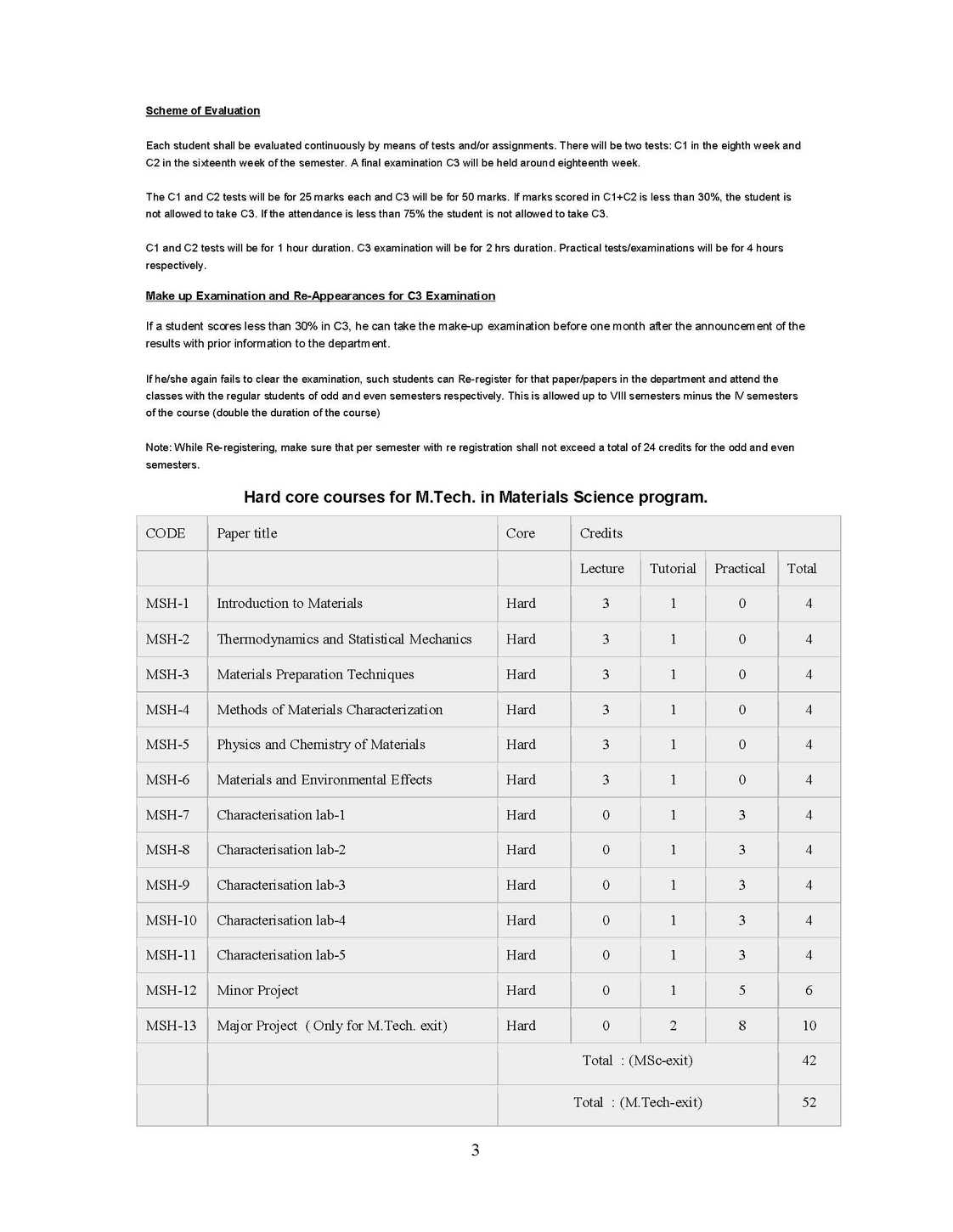 |