|
#1
April 20th, 2015, 12:47 PM
| |||
| |||
| NDA CTD Module 1
I want Module 1 Common Technical Document (CTD) format of choice for NDAs submitted to the FDA. What documents should have submitted by NDA to FDA? Is there any official link to submit all CTD, give me the procedure to submitting? Give me guidelines for Common Technical Document? Don’t worry I will get the Food and Drug Administration, NDA eCTD Technical Conformance Guide so that it would be easy for you to check module 1. Here are the content which come under file Introduction BACKGROUND PURPOSE DOCUMENT REVISION AND CONTROL RELATIONSHIP TO OTHER DOCUMENTS General Considerations ECTD PUBLISHING ECTD SAMPLES TRANSITIONING TO ECTD FORMAT AND RESUBMISSION OF NON-ECTD DOCUMENTS Transitioning from Paper to eCTD using us-regional v2.01 2.3.2 Transitioning from Paper to eCTD using us-regional v3.3 Transitioning from us-regional v2.01 to us-regional v3.3 Resubmission of non-eCTD documents ECTD LEAF TITLES ECTD LIFE CYCLE PRESUBMISSIONS ROLLING SUBMISSIONS STUDY TAGGING FILES 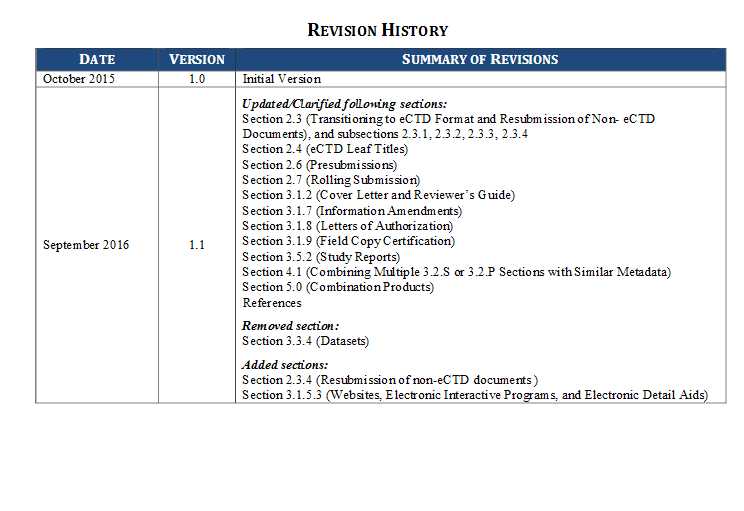  For full information please check the file Address:- U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Phone:- 1-888-INFO-FDA (1-888-463-6332) Last edited by Neelurk; June 18th, 2020 at 03:50 PM. |