|
#2
January 15th, 2016, 02:16 PM
| |||
| |||
| Re: PUC Karnataka state Chemistry Question Papers
As you have asked about the PUC Karnataka state Chemistry question papers, I am giving you some question below, Answer all the questions. 10x 1 =10 (Answer each question in one word or in one sentence) 1. State Law of definite Proportions . 2. Mention the type of intermolecular attractions that exists between non- polar molecules 3. H- is a Lewis base. Give reason. 4. Nitrogen has higher ionization enthalpy than that of Oxygen. Give reason. 5. What is the oxidation state of Mn in MnO4 6. Which alkali metal is the strongest reducing agent? 7. Solid carbon dioxide is also known as _________ 8. Mention the type of hybridization of carbon in diamond. 9. Mention one use of chromatography. 10.Draw the staggered conformation of ethane. Answer any FIVE questions (Each question carries two marks) 5x2=10 11.How many significant figures are in 0.2500 g? If the mass of one molecule of water is 18 u(amu), what is the mass of one mole of water molecules? 12.State Charles law. Give the relationship between density and molar mass of a gas. 13.Write the electronic configuration of H2 molecule. What is its bond order? 14.Differentiate between the reactions of Li and Na on burning them in oxygen. 15.What is the repeating unit in Organo Silicon polymer? Name the starting (raw) material used in the manufacture of Organo Silicon Polymer. - ? 17.Give two tests to distinguish between Alkanes and Alkenes. 18.How is Ozone layer formed in the stratosphere? Name a chief chemical that causes its depletion. Answer any FIVE questions (Each question carries three marks) 5x3=15 19.State modern periodic law and assign IUPAC name to the element with atomic number 114. Arrange the following in the decreasing order of their ionic radius:, Mg2+, Na+ N3- 20.Mention two conditions for the linear combination of atomic orbitals. Draw the shape of BMO formed by the LCAO of 1s and 1s atomic orbitals. 3 21.What are sigma and Pi bonds? Why is a sigma bond stronger than a Pi bond? 3 22.Define Dipole moment of a polar bond. Show that BeF2 molecule has zero dipole moment. 3 23.Balance the Redox reaction using oxidation number method: SO2 + H2S ææÆ S + H2O 3 PUC Karnataka state chemistry paper    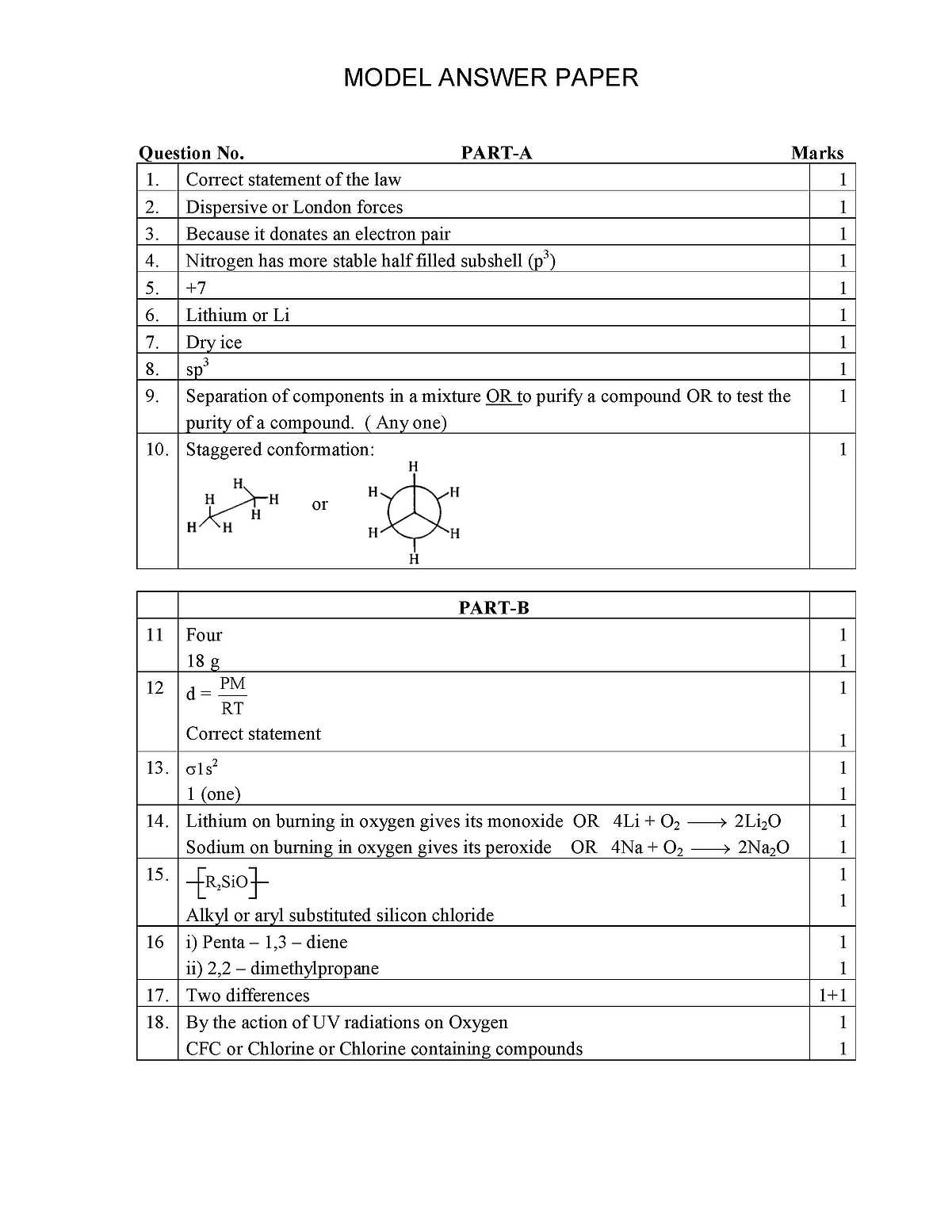   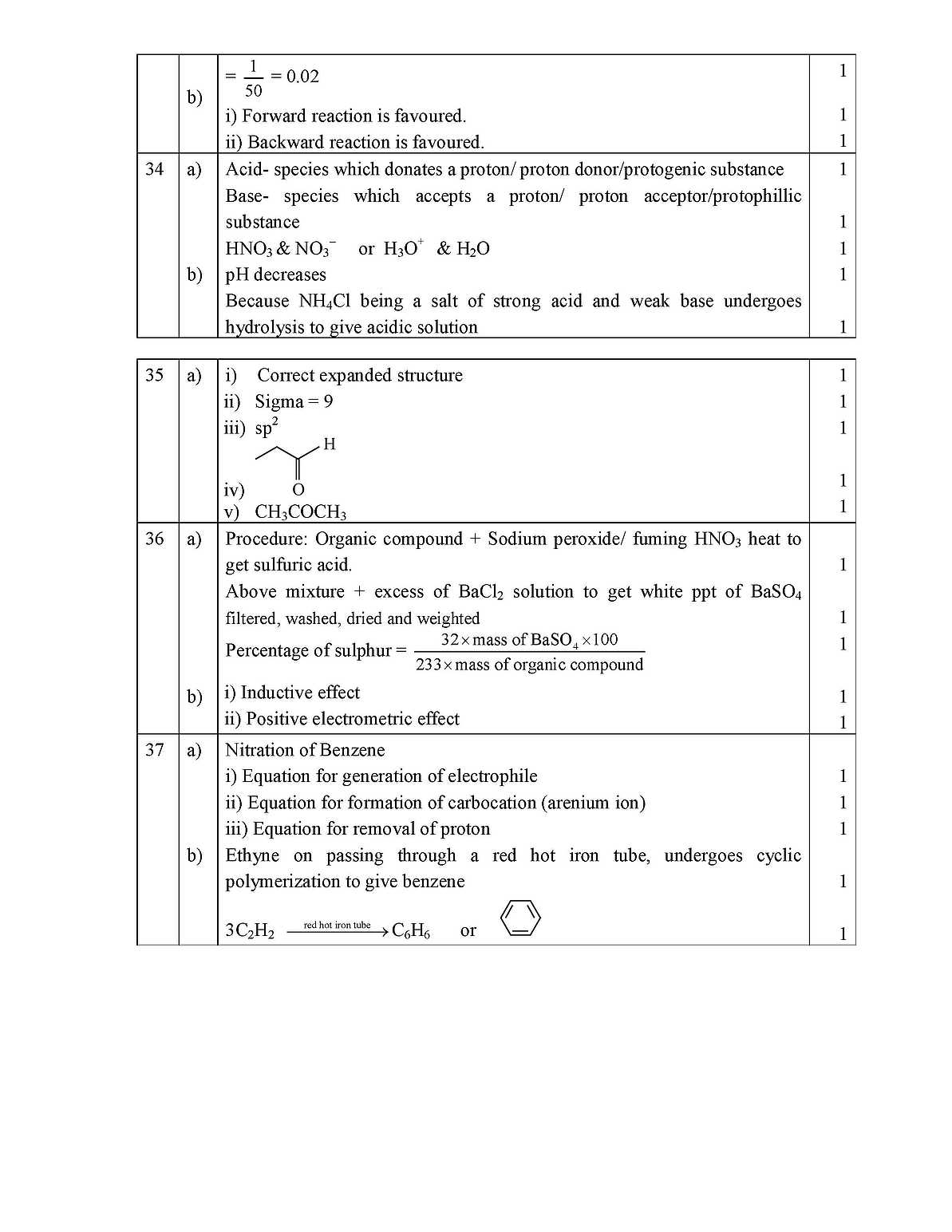 |