|
#1
June 1st, 2016, 03:35 PM
| |||
| |||
| Reaction of HCL and NAOH'
Guys , Would you please tell me How do I complete and balance an equation for Reaction of NaOH with HCl ? An Acid and Base always React to form a Salt and water, and it is called Neutralization. The salt formed in this reaction is sodium chloride, NaCl. The Equation for the Reaction is : NaOH + HCl = NaCl + H2O. The Numbers of Na, O, H and Cl atoms on the left and the right sides are as such equal. The Equation is already a balanced one. Balanced Reaction Between HCl and NaOH     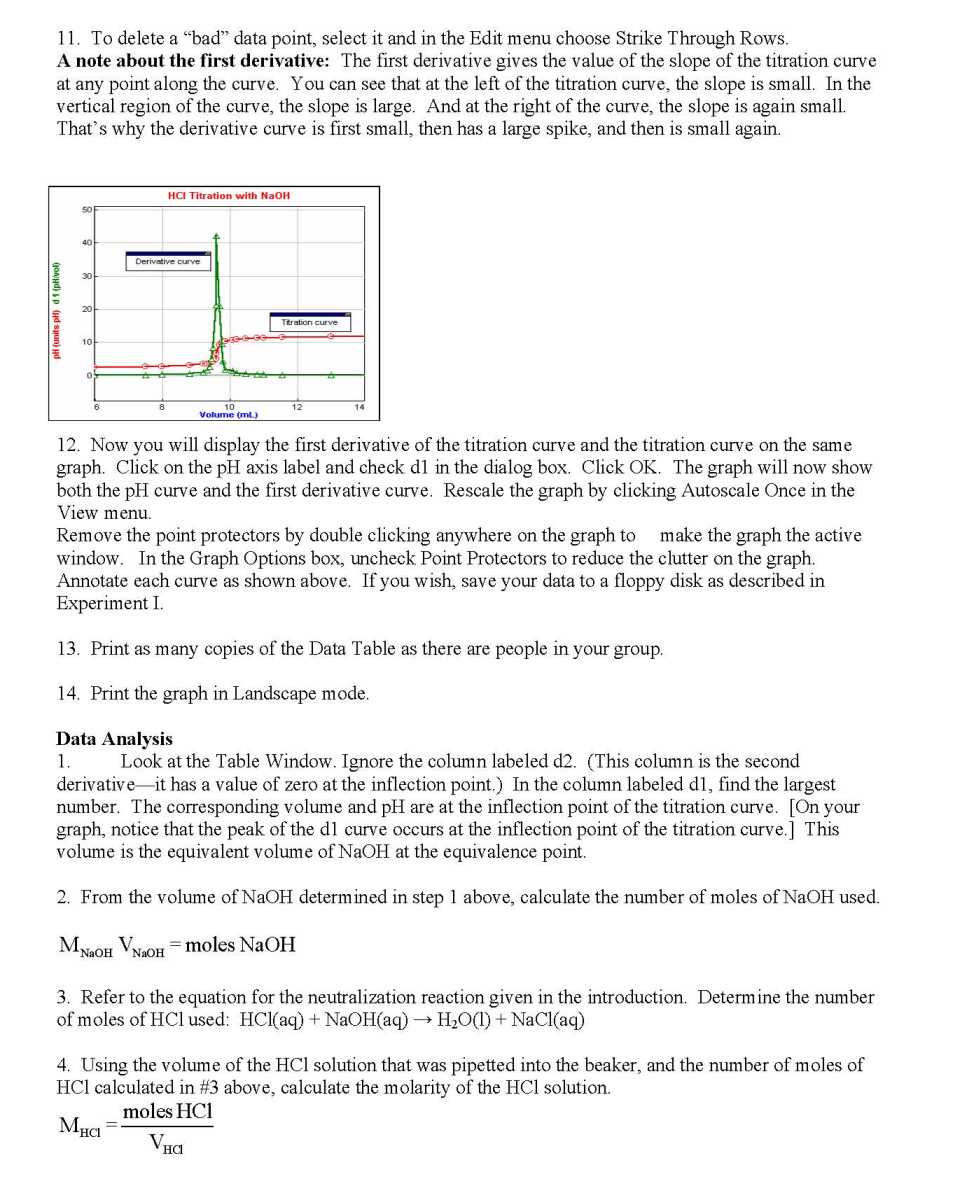   Last edited by Neelurk; June 10th, 2020 at 10:17 AM. |
| Similar Threads | ||||
| Thread | ||||
| Volume of HCL needed to Neutralize NAOH | ||||
| AL HCL Reaction | ||||
| HCL KMNO4 Reaction | ||||
| KOH + HCL Reaction | ||||
| Naoh + hcl | ||||