|
#2
March 23rd, 2017, 11:19 AM
| |||
| |||
| Re: Solved Question Papers of IIT JAM Chemistry
As you are looking for question papers of JAM Entrance Exam for admission in M.Sc Course at IIT, so here I am giving following paper: JAM Entrance Exam Question Paper for Chemistry Indicator used in redox titration is (A) Eriochrome black T (B) Methyl orange (C) Phenolphthalein (D) Methylene blue Among the following, the compound that has the lowest degree of ionic character is (A) NaCl (B) MgCl2 (C) AlCl3 (D) CaCl2 The coordination numbers of Cs+ and Cl– ions in the CsCl structure, respectively, are (A) 4,4 (B) 4,8 (C) 6,6 (D) 8,8 Determinant of a square matrix is always (A) a square matrix (B) a column matrix (C) a row matrix (D) a number The red color of ruby is due to (A) d-d transition of Cr3+ ion in Cr2O3 lattice (B) d-d transition of Cr3+ ion in Al2O3 lattice (C) ligand to metal charge transfer transition (D) metal to metal charge transfer transition The TRUE statement about [Cu(H2O)6]2+ is (A) All Cu–O bond lengths are equal (B) One Cu–O bond length is shorter than the remaining five (C) Three Cu–O bond lengths are shorter than the remaining three (D) Four Cu–O bond lengths are shorter than the remaining two At 298 K, 0.1 mol of ammonium acetate and 0.14 mol of acetic acid are dissolved in 1 L of water. The pH of the resulting solution is [Given: pKa of acetic acid is 4.75] (A) 4.9 (B) 4.6 (C) 4.3 (D) 2.3 The effective nuclear charge of helium atom is 1.7. The first ionization energy of helium atom in eV is (A) 13.6 (B) 23.1 (C) 39.3 (D) 27.2 JAM Entrance Exam Question Paper for Chemistry 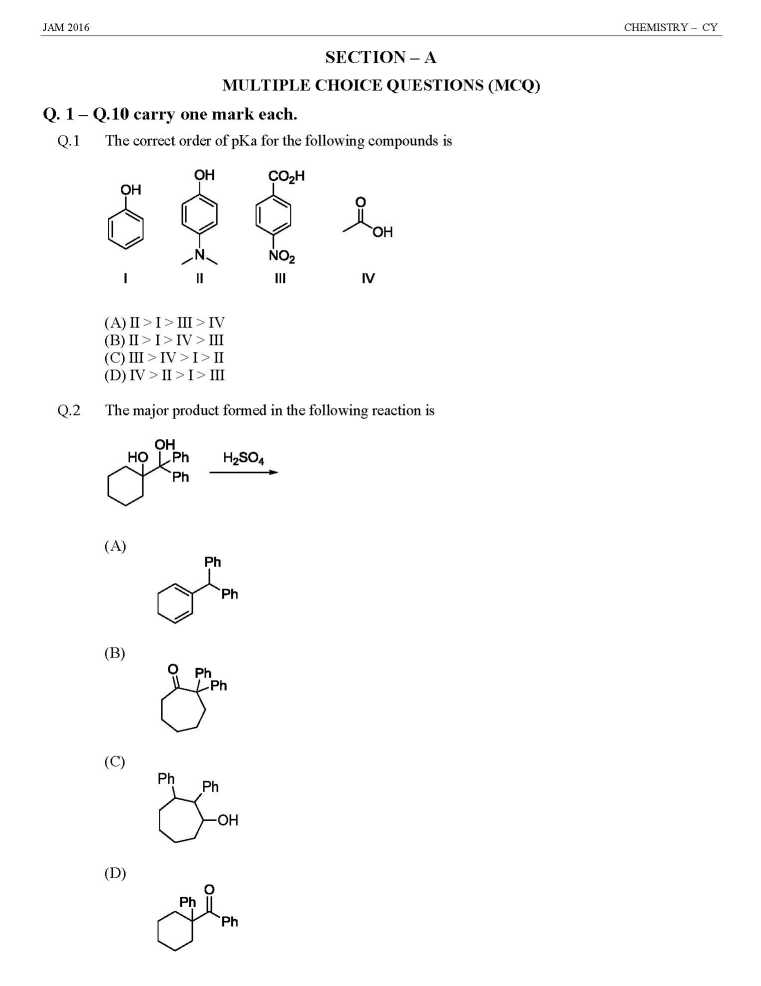 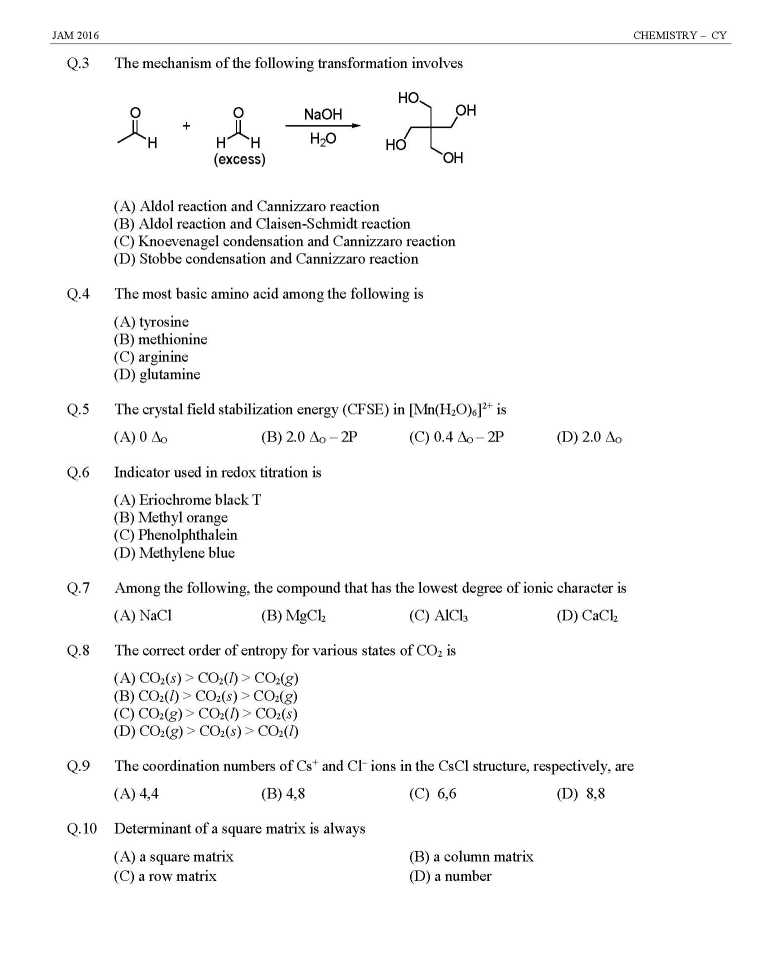 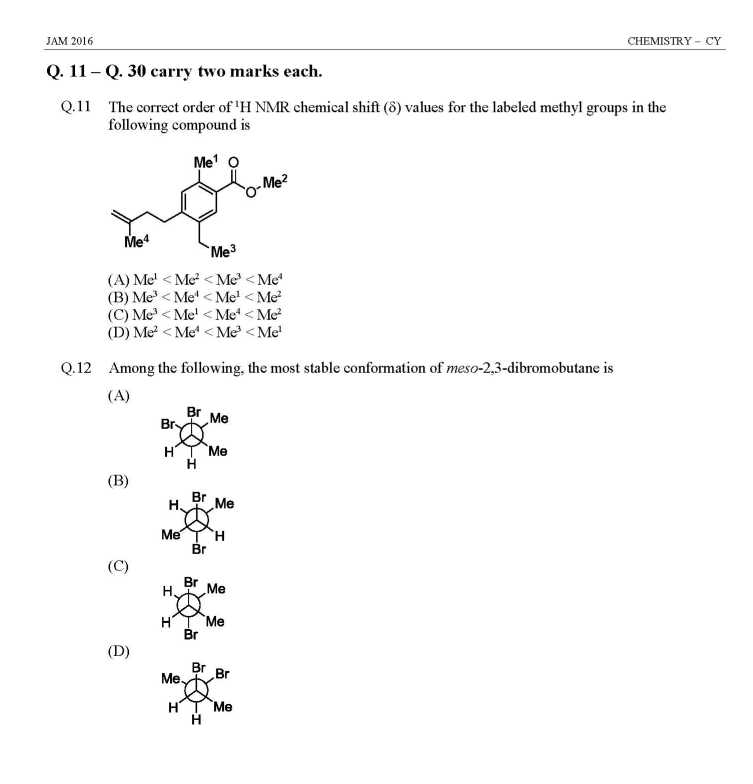 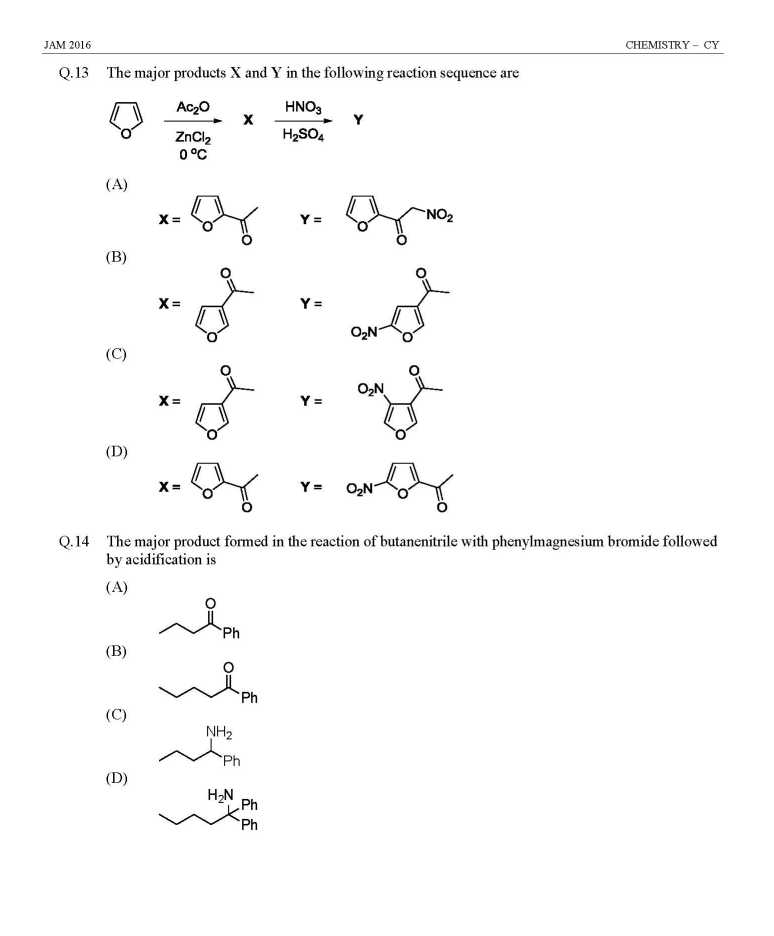 |