|
#2
May 5th, 2016, 03:25 PM
| |||
| |||
| Re: WBJEE Online MCQ Test
The West Bengal Joint Entrance Examinations Board was formed in the year 1962 for the purpose of holding Common Entrance Examinations for admission to the Undergraduate Level Engineering Courses in the State of West Bengal As per your demand here I am providing you previous year test paper of Chemistry In diborane, the number of electrons that account for bonding in the bridges is (A) Six (B) Two (C) Eight (D) Four A van der Waals gas may behave ideally when (A) The volume is very low (B) The temperature is very high (C) The pressure is very low (D) The temperature, pressure and volume all are very high The half-life for decay of 14C by β-emission is 5730 years. The fraction of 14C decays, in a sample that is 22,920 years old, would be (A) 1/8 (B) 1/16 (C) 7/8 (D) 15/16 2-Methylpropane on monochlorination under photochemical condition give (A) 2-Chloro-2-methylpropane as major product (B) (1:1) Mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane (C) 1-Chloro-2-methylpropane as a major product (D) (1:9) Mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane For a chemical reaction at 27°C, the activation energy is 600 R. The ratio of the rate constants at 327°C to that of at 27°C will be (A) 2 (B) 40 (C) e (D) e2 Chlorine gas reacts with red hot calcium oxide to give (A) Bleaching powder and di hlorine monoxide (B) Bleaching powder and water (C) Calcium chloride and chlorine dioxide (D) Calcium chloride and oxygen Ans : (D) Acid catalysed hydrolysis of ethyl acetate follows a pseudo-first order kinetics with respect to ester. If the reaction is carried out with large excess of ester, the order wi h respect to ester will be (A) 1.5 (B) 0 (C) 2 (D) 1 16. The different colours of litmus in acidic, neutral and basic solutions are, respectively (A) Red, orange and blue (B) Blue, violet and red (C) Red, colourless and blue (D) Red, violet and blue Baeyer’s reagent is (A) Alkaline potassium permanganate (B) Acidified potassium permanganate (C) Neutral potassium permanganate (D) Alkaline potassium manganate Nitric acid can be obtained from ammonia via the formations of the intermediate compounds (A) Nitric oxides and nitrogen dioxides (B) Nitrogen and nitric oxides (C) Nitric oxide and dinitrogen pentoxide (D) Nitrogen and nitrous oxide  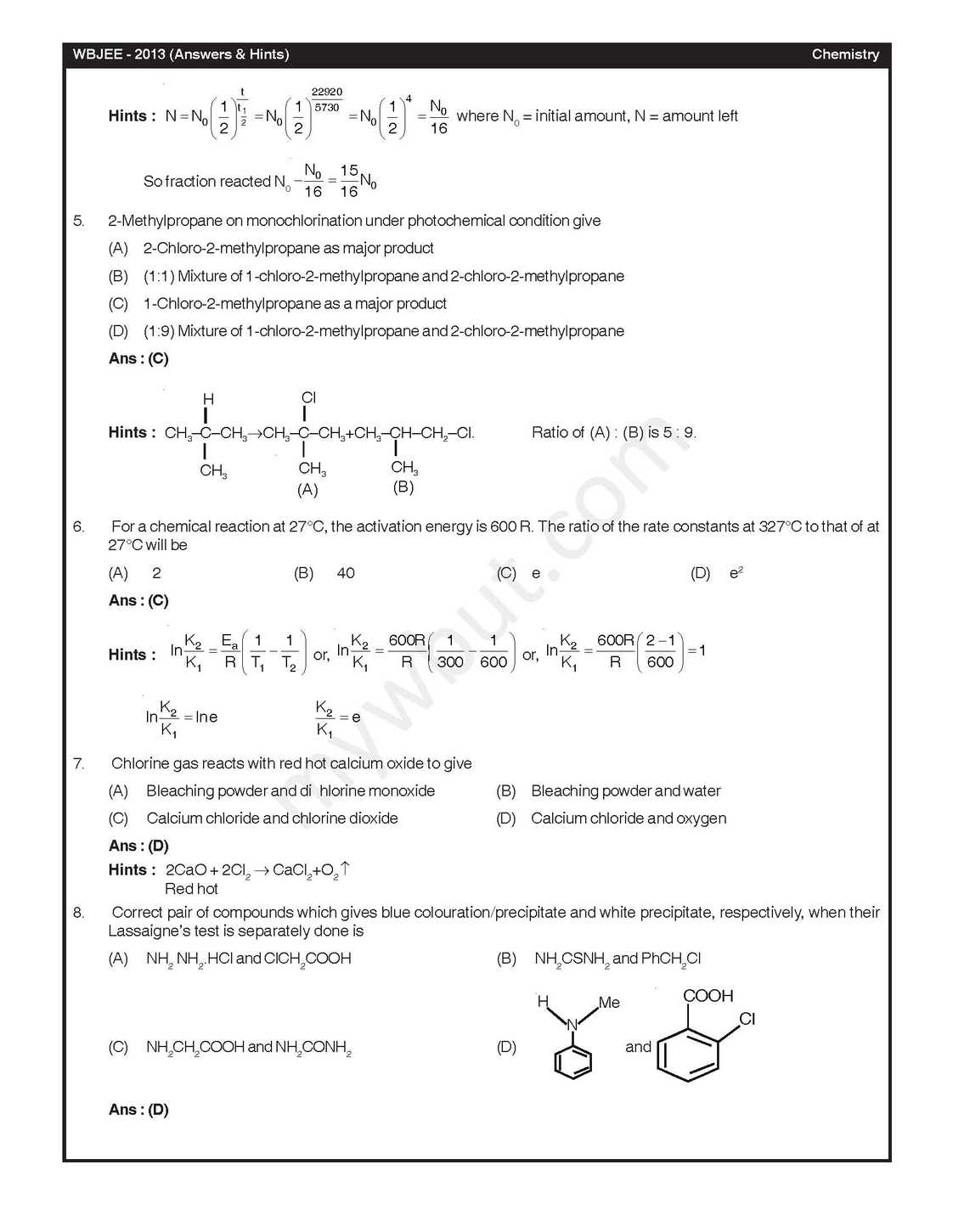 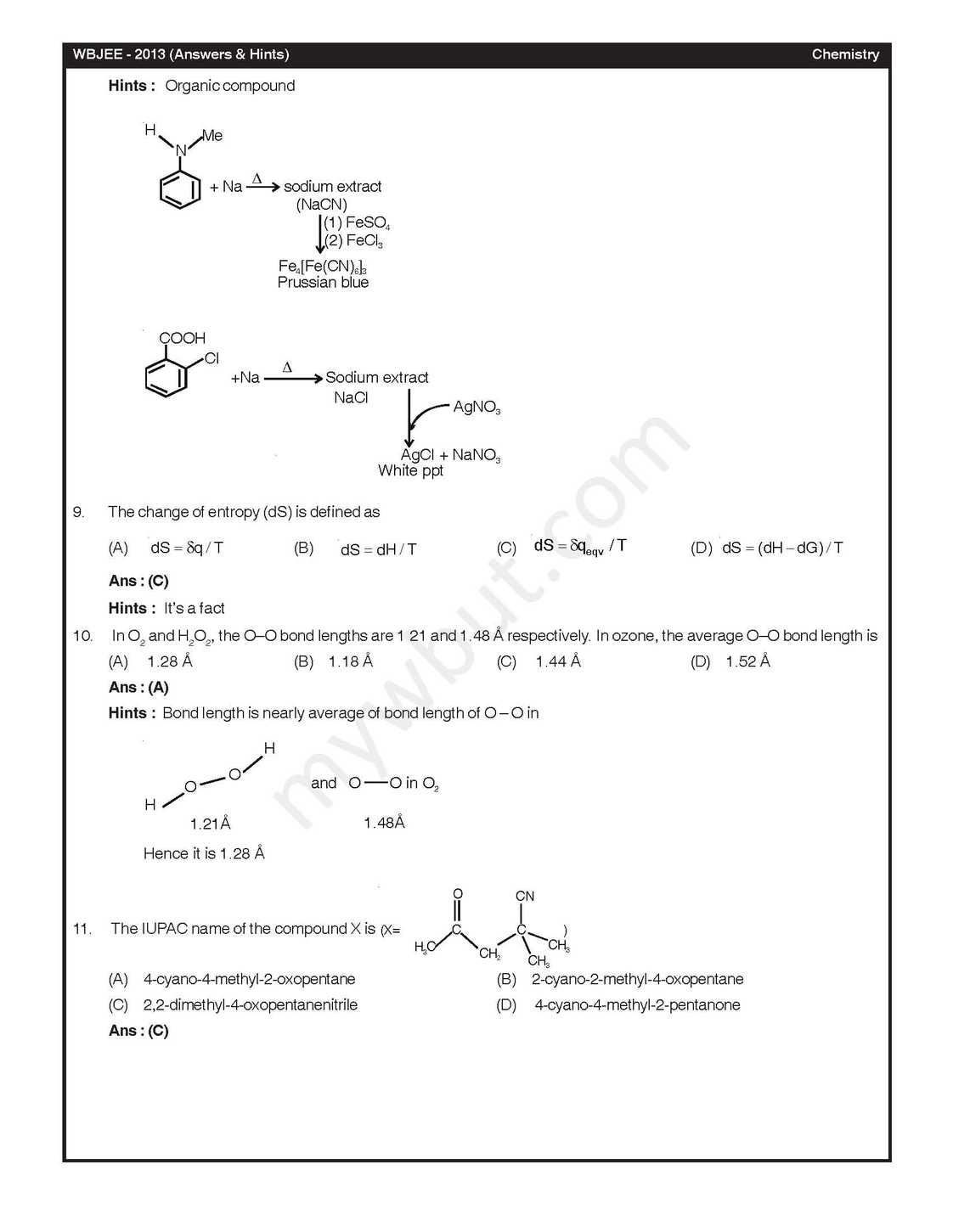 For complete paper here I am attaching a file: |