|
#1
June 4th, 2016, 09:41 AM
| |||
| |||
| AICTE GPAT Answer Key
Hey will you please give me answer key of AICTE GPAT exam? My brother is looking for AICTE GPAT Answer Key so please provide me? Graduate Pharmacy Aptitude Test (GPAT) is a national level entrance exam conducted by All India Council for Technical Education (AICTE) every year as per the directions of Ministry of Human Resource Development (MHRD), Government of India. As per my searching I was not founded AICTE GPAT Answer Key. You can get AICTE GPAT Answer Key after visiting site of AICTE: 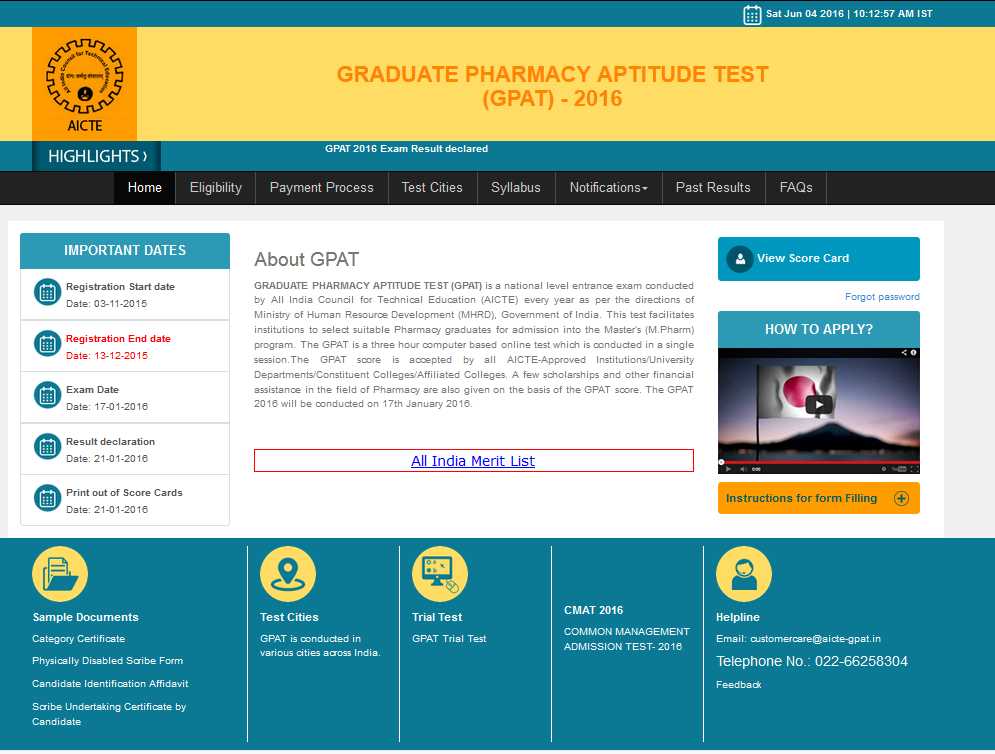 Eligibility Criteria Candidates must be a citizen of India Candidates must be Bachelor’s degree holders in Pharmacy (4 years after 10+2, including lateral entry candidates) Syllabus of AICTE GPAT PHARMACEUTICS Introduction to Physical pharmacy •Matter, Properties of Matter: State of matter, change in the state of matter, latent heats and vapor pressure, sublimation critical point, Eutectic mixtures, gases, aerosols-inhalers, relative humidity, liquid. Complexes, liquid crystals, glassy state, solids- crystalline, amorphous and polymorphism. •Micromeretics and Powder Rheology: Particle size and distribution, average particle size, number and weight distribution, particle number, methods for determining particle volume, methods of determining particle size- optical microscopy, sieving, sedimentation; measurements of particle shape, specific surface area; methods for determining surface area; permeability, adsorption, derived properties of powders, porosity, packing arrangement, densities, bulkiness & flow properties. •Surface and Interfacial Phenomenon: Liquid interface, surface and interfacial tensions, surface free energy, measurement of surface and interfacial tensions, spreading coefficient, adsorption at liquid interfaces, surface active agents, HLB classification, solubilization, detergency, adsorption at solid interfaces, solid-gas and solid-liquid interfaces, complex films, electrical properties of interface. •Viscosity and Rheology: Newtonian systems, Law of flow, kinematic viscosity, effect of temperature; non-Newtonian systems: pseudoplastic, dilatant, plastic; thixotropy, thixotropy in formulation, negative thixotropy, determination of viscosity, capillary, falling ball, rotational viscometers. •Dispersion Systems: Colloidal dispersions: Definition, types, properties of colloids, protective colloids, applications of colloids in pharmacy; Suspensions and Emulsions: Interfacial properties of suspended particles, settling in suspensions, theory of sedimentation, effect of Brownian motion, sedimentation of flocculated particles, sedimentation parameters, wetting of particles, controlled flocculation, flocculation in structured vehicles, rheological considerations; Emulsions-types, theories, physical stability. •Complexation: Classification of complexes, methods of preparation, analysis, & applications. •Kinetics and Drug Stability: General considerations & concepts, half-life determination, Influence of temperature, light, solvent, catalytic species and other factors, Accelerated stability study, expiration dating. Importance of microbiology in pharmacy •Structure of bacterial cell; Classification of microbes and their taxonomy: Actinomycetes, bacteria, rickettsiae, spirochetes and viruses. •Identification of Microbes: Stains and types of staining techniques, electron microscopy; Nutrition, cultivation, isolation of bacteria, actinomycetes, fungi, viruses, etc; microbial genetics and variation. •Control of microbes by physical and chemical methods: Disinfection, factors influencing disinfectants, dynamics of disinfection, disinfectants and antiseptics and their evaluation. •Sterilization: Different methods, validation of sterilization methods &equipments; Sterility testing of all pharmaceutical products. Microbial assays of antibiotics, vitamins & amino acids. •Immunology and Immunological Preparations: Principles, antigens and heptans, immune system, cellular/humoral immunity, immunological 2 tolerance, antigen-antibody reactions and their applications. Hypersensitivity, active and passive immunization. Vaccines and sera: their preparation, standardization and storage. •Genetic Recombination: Transformation, conjugation, transduction, protoplast fusion and gene cloning and their applications. Development of hybridoma for monoclonal antibodies. Study of drugs produced by biotechnology such as Activase, Humulin, Humatrope, HB etc. •Antibiotics: Historical development of antibiotics. Antimicrobial spectrum and methods used for their standardization. Screening of soil for organisms producing antibiotics, fermenter, its design, control of different parameters. Isolation of mutants, factors influencing rate of mutation. Design of fermentation process. Isolation of fermentation products with special reference to penicillins, streptomycins, tetracyclines and vitamin B12. Introduction to pharmaceutical jurisprudence & ethics •Pharmaceutical Legislations: A brief review; Drugs & Pharmaceutical Industry - A brief review; Pharmaceutical Education •An elaborate study of the followings: Pharmaceutical Ethics; Pharmacy Act 1948; Drugs and Cosmetics Act 1940 and Rules 1945; Medicinal & Toilet Preparations (Excise Duties) Act 1955; Narcotic Drugs & Psychotropic Substances Act 1985 & Rules; Drugs Price Control Order. •A brief study of the following Acts with special reference to the main provisions and the latest amendments: Poisons Act 1919; Drugs and Magic Remedies (Objectionable Advertisements) Act 1954; Medical Termination of Pregnancy Act 1970 & Rules 1975; Prevention of Cruelty to Animals Act 1960; States Shops & Establishments Act & Rules; Insecticides Act 1968; AICTE Act 1987; Factories Act 1948; Minimum Wages Act 1948; Patents Act 1970. A brief study of the various Prescription/Non-prescription Products. Medical/Surgical accessories, diagnostic aids, appliances available in the market. 3 Introduction to dispensing and community pharmacy •Prescription: Handling of prescription, source of errors in prescription, care required in dispensing procedures including labeling of dispensed products. General dispensing procedures including labeling of dispensed products; Pharmaceutical calculations: Posology, calculation of doses for infants, adults and elderly patients; Enlarging and reducing recipes percentage solutions, alligation, alcohol dilution, proof spirit, isotonic solutions, displacement value etc. •Principles involved and procedures adopted in dispensing of: Typical prescriptions like mixtures, solutions, emulsions, creams, ointments, powders, capsules, pastes, jellies, suppositories, ophthalmic, pastilles, lozenges, pills, lotions, liniments, inhalations, paints, sprays, tablet triturates, etc. •Incompatibilities: Physical and chemical incompatibilities, inorganic incompatibilities including incompatibilities of metals and their salts, non-metals, acids, alkalis, organic incompatibilities. Purine bases, alkaloids, pyrazolone derivatives, amino acids, quaternary ammonium compounds, carbohydrates, glycosides, anesthetics, dyes, surface active agents, correction of incompatibilities. Therapeutic incompatibilities. •Community Pharmacy: Organization and structure of retail and whole sale drug store-types of drug store and design, legal requirements for establishment, maintenance and drug store-dispensing of proprietary products, maintenance of records of retail and wholesale, patient counseling, role of pharmacist in community health care and education (First aid, communicable diseases, nutrition, family planning). •Organization and Structure of hospital pharmacy: Organization of a hospital and hospital pharmacy, Responsibilities of a hospital pharmacist, Pharmacy and therapeutic committee, Budget preparation and Implementation. 4 •Hospital Formulary: Contents, preparation and revision of hospital formulary. •Drug Store Management and Inventory Control: Organization of drug store, Types of materials stocked, storage conditions; Purchase and Inventory Control principles, purchase procedures, Purchase order, Procurement and stocking. •Drug distribution Systems in Hospitals: Out-patient dispensing, methods adopted; Dispensing of drugs to in-patients. Types of drug distribution systems. Charging policy, labeling; Dispensing of drugs to ambulatory patients; Dispensing of controlled drugs, Dispensing of ancillary supplies. •Central Sterile Supply Unit and their Management: Types of materials for sterilization, Packing of materials prior to sterilization, sterilization equipments, Supply of sterile materials. •Manufacture of Sterile and Non-sterile Products: Policy making of manufacturable items, demand and costing, personnel requirements, manufacturing practice, Master formula Card, production control, Manufacturing records. •Drug Information Services: Sources' of Information on drugs, disease, treatment schedules, procurement of information, Computerized services (e.g., MEDLINE), Retrieval of information, Medication error- types of medication errors, correction and reporting. Important Dates: Registration Start date Date: 03-11-2015 Registration End date Date: 13-12-2015 Exam Date Date: 17-01-2016 Result declaration Date: 21-01-2016 Print out of Score Cards Date: 21-01-2016 Here I’m attaching PDF of AICTE GPAT: Last edited by Neelurk; June 18th, 2020 at 10:40 AM. |