|
#2
March 18th, 2017, 10:25 AM
| |||
| |||
| Re: HCL PKA Value
The best place to begin is at acids – and as we probably am aware acids can surrender a proton to end up deprotonated.  Acid and Conjugate Base (and charges) The corrosive will separate into a proton (H+) and cunjugate base (A-). Take note of this is in balance – there is a blend of both sides of the condition display. The consistent for this equilibium (the corrosive separation steady, Ka) reveals to us the position of the harmony. Ka is the grouping of the items over the convergence of the reagents: 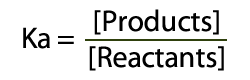 Ka = Products Concentrations / Reagent Concentrations in this way, for Hydrochloric Acid (HCl):  Corrosive Dissociation Constant for HCl To outline, a solid corrosive like HCl has a Ka estimation of 1×10^7, which obviously demonstrates a huge inclination towards items. Acidic corrosive then again has a Ka estimation of 1.7×10^-5 – which unequivocally supports reagents. Anyway, what's this "log" stuff? We utilize logs to change over long numbers into an easy to use scale – as the numbers we regularly get are on a tremendous scale (see HCl and Acetic Acid above). To do this we place p into our corrosive separation consistent Ka. 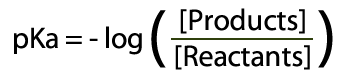 pKa and Log of the Concentrations The ionisation of HCl can be shown as: HCl+H2O→H3O++Cl− Ka can be calculated as the ratio of the product of the concentrations of the products to that of the reactant, that is, Ka=[H3O+][Cl−][HCl] Since HCl is a strong acid, the value of Ka turns out to be very large, that is, Ka=1071=107 (approx.) The value of pKa is given by pKa=−logKa pKa=−(log107) pKa=−(7)=−7 |