|
#1
June 7th, 2016, 10:56 AM
| |||
| |||
| Ka of HCL
Hi I would like to have information about what is KA generally mean and what is the value of Ka for HCL? Ka, in old Egyptian religion, with the ba and the akh, a main part of the spirit of an individual or of a divine being. The careful centrality of the ka remains a matter of discussion, essentially for absence of an Egyptian definition; the standard interpretation, "twofold," is off base. Actually it doesn't make a difference in watery arrangement, since everything will be changed over into hydronium and chloride particle , that is the reason we compose the response in one bearing as it were. HCl (aq) ==> H+ + Cl- The corrosive is 100% separated, so each and every last particle of HCl will be changed over into H+ and Cl-. For solid acids in the gas stage it makes a distinction, however in the event that you are managing fluid arrangements, discussing Ka values for solid acids is immaterial. That is the reason for any solid corrosive, the pH of the arrangement is - log([strong acid]), so arrangements of 0.1 M HCl, HBr, HI, HNO3 and HClO4, all will give you the same pH, 1.0. Any solid corrosive when broken down in water is changed over into H+, since H+ is the most grounded corrosive that can exist in water. That is known as the leveling impact. Generally, utilizing this methodology, you can say that the Ka of any solid corrosive methodologies interminability, any number isolated by zero is basically boundlessness. Please find the list for the Table of Acid and Base Strength which also has their Ka value: 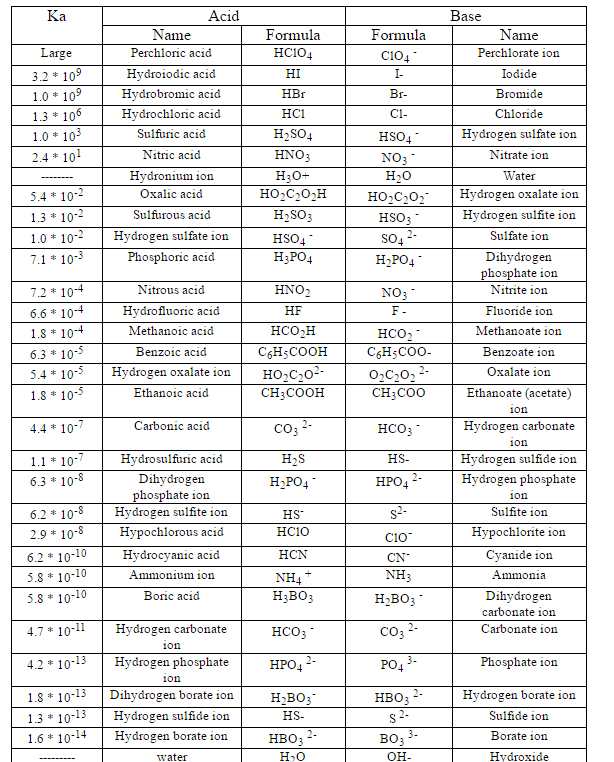 Last edited by Neelurk; March 2nd, 2020 at 02:57 PM. |