|
#1
October 9th, 2017, 04:03 PM
| |||
| |||
| RFM of HCL
Hi I would like to have the information about Relative Formula Mass (RFM) as well as Relative Atomic Mass and the details of the Molecular weight and also the Molecular Weight of HCL? The Relative Formula Mass (RFM) is simply just a sum of the atomic masses that make up the compound in question. RFM Absorbers 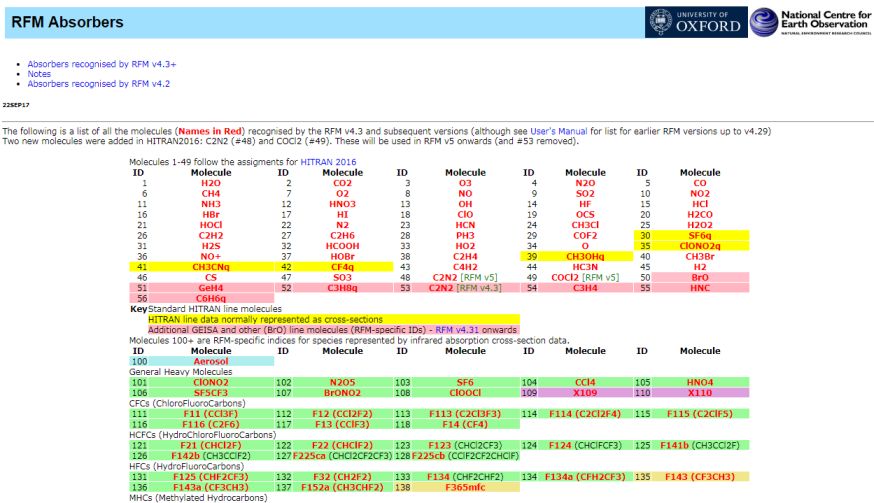 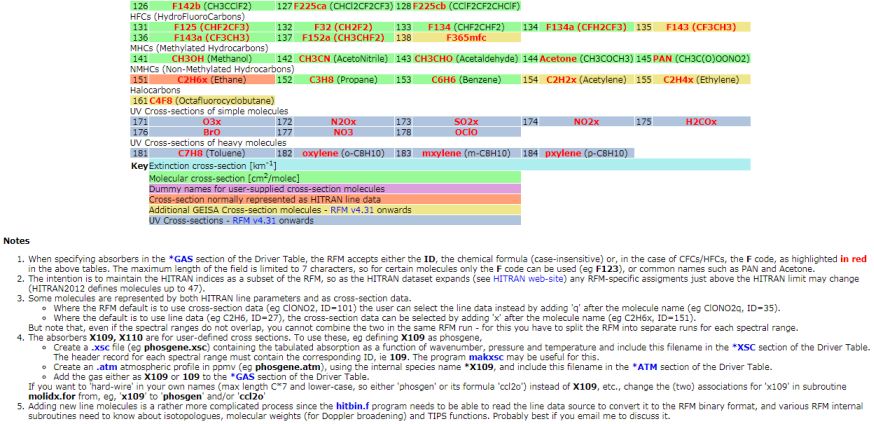 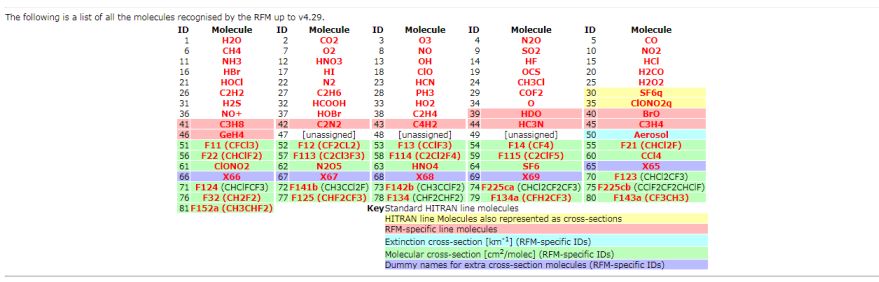 Relative Atomic Mass The Relative Atomic Mass of a substance component gives us a thought of how overwhelming it feels (the power it makes when gravity pulls on it). In the event that you take a gander at the occasional table you can discover the relative nuclear masses of the components. The number at the highest point of the image is the Relative Atomic Mass (Ar ) Molecular Weight In chemistry, the recipe weight is an amount figured by duplicating the nuclear weight (in nuclear mass units) of every component in a compound equation by the quantity of molecules of that component introduce in the recipe, at that point including these items together. Discovering molar mass begins with units of grams per mole (g/mol). While figuring atomic weight of a concoction compound, it discloses to us what number of grams are in one mole of that substance. The equation weight is basically the weight in nuclear mass units of the considerable number of iotas in a given recipe. A typical demand on this site is to change over grams to moles. To finish this figuring, you need to realize what substance you are attempting to change over. The reason is that the molar mass of the substance influences the transformation. This site discloses how to discover molar mass. Utilizing the synthetic equation of the compound and the occasional table of components, one can include the nuclear weights and figure atomic weight of the substance. On the off chance that the recipe utilized as a part of figuring molar mass is the atomic equation, the recipe weight registered is the sub-atomic weight. The rate by weight of any iota or gathering of particles in a compound can be processed by partitioning the aggregate weight of the molecule (or gathering of iotas) in the recipe by the equation weight and increasing by 100. Recipe weights are particularly helpful in deciding the relative weights of reagents and items in a concoction response. These relative weights processed from the compound condition are at times called condition weights. Molecular weight of Hydrochloric Acid Molar mass of HCl = 36.46094 g/mol Last edited by Neelurk; May 8th, 2020 at 11:43 AM. |