|
#2
March 21st, 2017, 12:19 PM
| |||
| |||
| Re: Conjugate Base of HCL
A conjugate acid, inside the Brønsted–Lowry acid–base hypothesis, is a species framed by the gathering of a proton (H+) by a base—as it were, it is a base with a hydrogen particle added to it. Then again, a conjugate base is simply what is left after a corrosive has given a proton in a synthetic response. Consequently, a conjugate base is an animal groups shaped by the expulsion of a proton from a corrosive. In rundown, this can be spoken to as the accompanying concoction response: Corrosive + Base ⇌ Conjugate Base + Conjugate Acid Corrosive base responses In a corrosive base response, a corrosive in addition to a base responds to shape a conjugate base in addition to a conjugate corrosive: Conjugates are shaped when a corrosive loses a hydrogen proton or a base picks up a hydrogen proton. Allude to the accompanying figure: 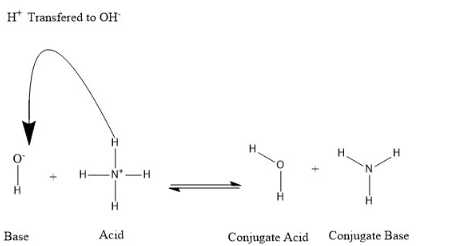 Pour HCl in water and write the equation: HCl + H2O ---> H3O+ + Cl- The acid is HCl, and the conjugate base is Cl-. The name of the base is chloride ion. |