|
#2
June 6th, 2016, 08:51 AM
| |||
| |||
| Re: PH Base Indicator
pH Indicators also known as Acid - Base Indicators A pH indicator is a Halochromic Chemical Compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually. Acid - Base Indicators: The most common method to get an idea about the pH of solution is to use an acid base indicator. An indicator is a large organic molecule that works somewhat like a " color dye". The Most Common Indicator is Found on "Litmus" Paper. It is red below pH 4.5 and blue above pH 8.2. Color Blue Litmus Red Litmus Acid turns red stays same Base stays same turns blue The Weak acid Form (HIn) will have one Color and the weak acid negative ion (In-) will have a different color. The weak acid equilibrium is: HIn → H+ + In- For phenolphthalein: pH 8.2 = colorless; pH 10 = red For bromophenol blue: pH 3 = yellow; pH 4.6 = blue Graphic Colors and pH Ranges. 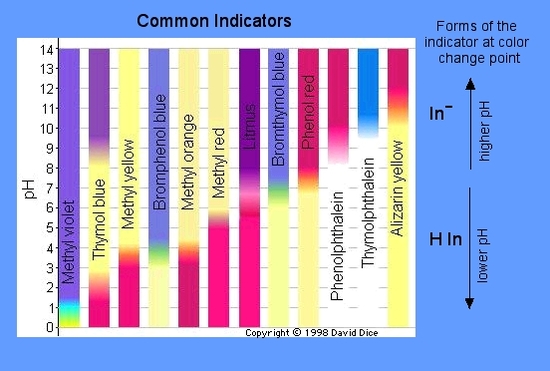 : : Reaction Equilibrium: HIn → H+ + In- colorless red The equilibrium shifts right, HIn decreases, and In- increases. As the pH increase between 8.2 to 10.0 the color becomes red because of the equilibrium shifts to form mostly In- ions. |