|
#2
June 28th, 2016, 12:13 PM
| |||
| |||
| Re: AIEEE PDF Download
As you have asked for the All India Engineering Entrance Exam (AIEEE) Previous year paper, I am providing you with it, check below for the details The standard enthalpy of formation of NH3 is -46.0 kJ mol-1. If the enthalpy of formation of H2 from its atoms is -436 kJ mol-1 and that of N2 is -712 kJ mol-1, the average bond enthalpy of N-H A •nNH3 is (1) -964 kJ mol-1 (2) +352 kJ mol-1 (3) + 1056 kJ mol-1 (4) -1102 kJ m The time for half life period of a certain reaction A ---+ prod ts s hour. When the initial concentration of the reactant 'A', is 2.0 mol L -\ how much ti o e for its concentration to come from 0. 50 to 0. 25 mol L -1 if it is a zero order reaction ? (1) 4h (2) 0.5h (3) 0.25 (4) 1 h A solution cont(t(ling 2.675 g of CoCI3. 6 NH3 (molar mass = 267.5 g mol- 1) is passed through acation exch t~ Jhe hloride ions obtained in solution were treated with excess of AgN03 to give 4. 78 g of g r mass = 143.5 g mol- \ The formula of the complex is (At. Mass of Ag = 1 08 u) (1) [Co (2) [CoCI2(NH3)4]CI (3) [CoCI3(NH3)3] (4) [CoCI(NH3)s]CI2 For more details, you can refer to the attached file AIEEE Previous year paper  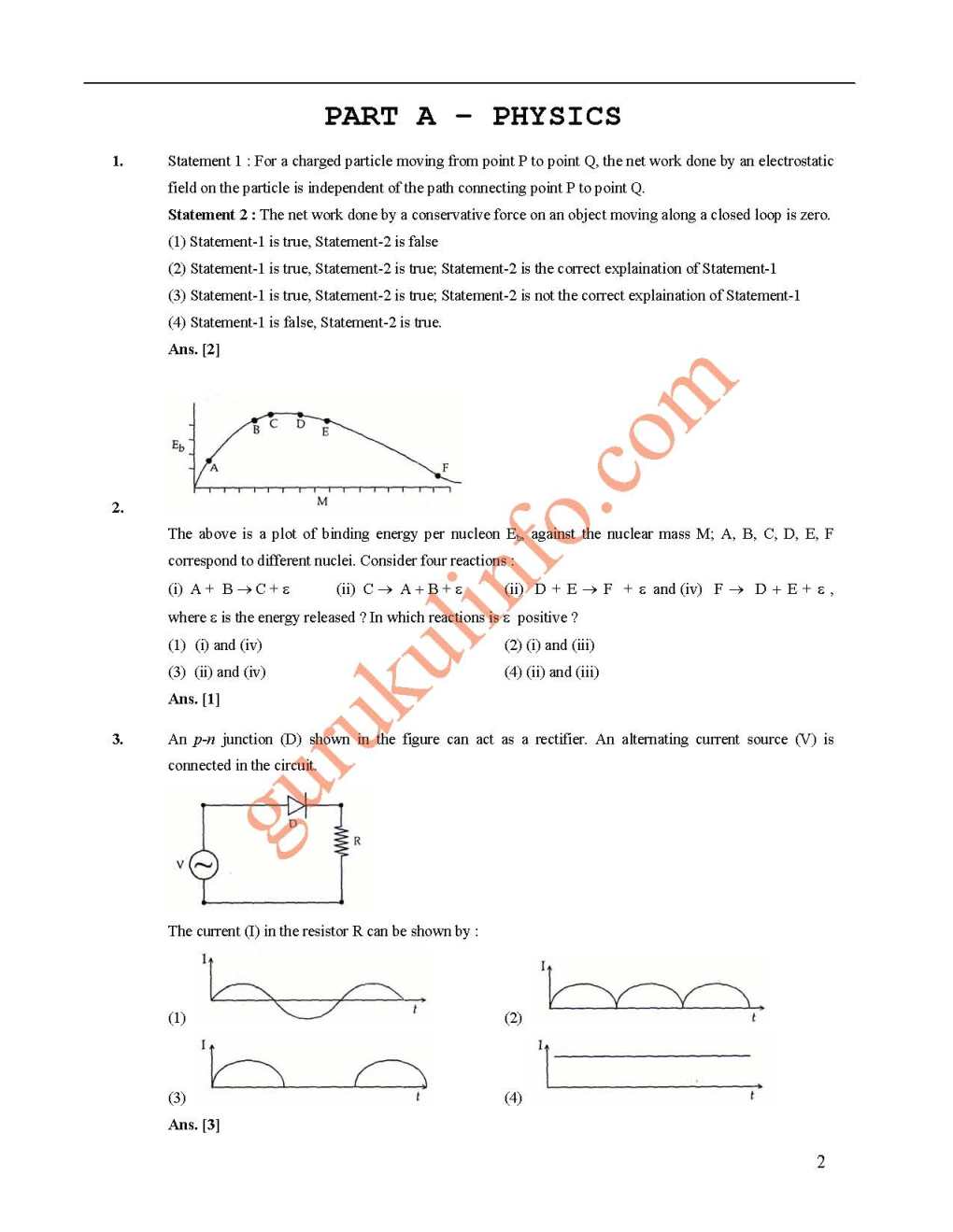 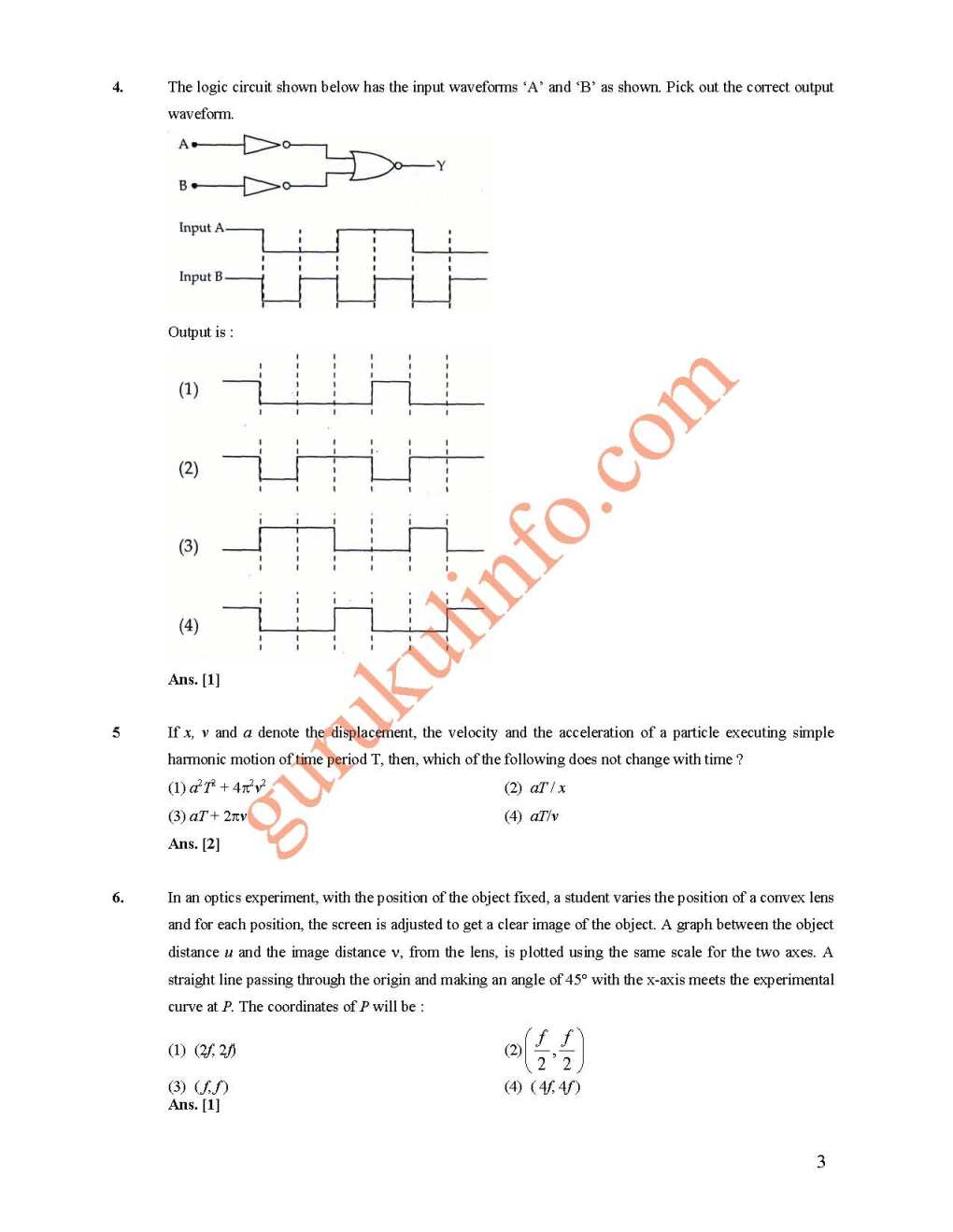  |